Washington, D.C. — The U.S. Supreme Court (SCOTUS) has agreed to take up two controversial court cases related to the U.S. Food and Drug Administration’s (FDA) removal of critical safeguards since its initial approval of mifepristone. Mifepristone is the first of a two-drug regimen that kills the in utero child by withholding life-sustaining hormones.
The High Court will hear oral arguments in the upcoming election year.
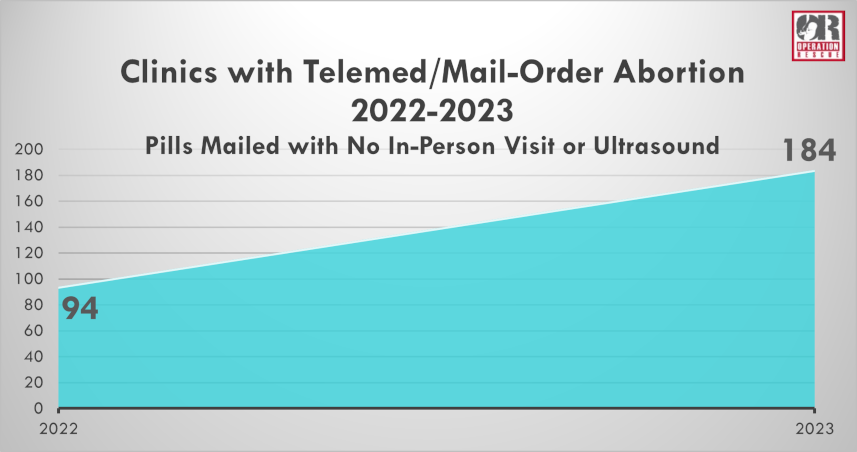
Operation Rescue’s recently released annual survey revealed that the FDA’s removal of these crucial protections, otherwise known as Risk Evaluation and Mitigation Strategies, for women who take the drugs has proven to result in the widespread increase of dangerous mail-order abortions.
Brick-and-mortar abortion facilities that mail pills without an in-clinic visit nearly doubled from 2022 to 2023. And a total of 13 new back-alley, online abortion pill suppliers came into existence.
According to the SCOTUS docket, the two closely related cases below will be consolidated, and a total of one hour will be allotted for oral argument. In other words, SCOTUS will hear both cases as one.
- Food and Drug Administration v. Alliance for Hippocratic Medicine, 23-235.
- Danco Laboratories v. Alliance for Hippocratic Medicine, 23-236.
The two appeals above originated from the November 2022 lawsuit, Alliance for Hippocratic Medicine (AHM) v. U.S. Food and Drug Administration (FDA). In the first case, the FDA is appealing a Fifth Circuit Court Ruling that partially upheld an April 7 ruling by U.S. District Judge Matthew Kacsmaryk. In the second case, Danco Laboratories, the manufacturer of Mifeprex, the name-brand version of mifepristone, is appealing the same decision. The arguments heard will now specifically address the possibiity of reinstating 2011 safeguards that limited use of mifepristone to seven weeks of pregnancy and prohibited distribution by mail.
In Review: Actions Related to the ‘Abortion Pill’
2000: FDA’s Approved Mifepristone with Necessary Safeguards
Mifepristone was originally approved in 2000 with the following safety requirements:
- Physician Supervision: A physician must supervise administration of the drug in order to “date pregnancies and diagnose ectopic pregnancies.” Though specific testing wasn’t identified, trained physicians were expected to rule out risk factors that would make women clinically ineligible.
- In-Person Follow-Up: An in-person follow-up appointment must take place to protect women from sepsis should the remains of the child remain inside her body after his or her death.
- Only First Seven Weeks of Pregnancy: Administration of the drug is limited to seven weeks of pregnancy, ensuring the abortion takes place early in pregnancy.
2016: FDA Removed Most Safeguards, Expanded Access
In 2016, crucial safeguards were removed, expanding accessibility by allowing for the drug to be:
- Prescribed to pregnant women three weeks further along in pregnancy (up to 10 weeks),
- Prescribed and administered by non-physicians,
- Prescribed and administered without an in-person follow-up appointment.
2021: FDA Temporarily Removed Initial In-Person Consultation Requirement
In 2021, during the COVID-19 pandemic, the in-person consultation requirement for administration of the drug was temporarily suspended.
2023: Battle Over Back-Alley Abortions Intensifies
In 2023, the FDA abandoned nearly every remaining safety precaution by:
- Making the temporary suspension of the in-person consultation requirement permanent (January 2023).
- Changing the label of mifepristone to allow retail pharmacies to distribute the drug (also January 2023).
Additional 2023 Timeline Concerning 2024 SCOTUS Case
April 7: U.S. District Judge Matthew Kacsmaryk ruled in the Alliance for Hippocratic Medicine (AHM) v. U.S. Food and Drug Administration (FDA) case to suspend the FDA’s approval of mifepristone.
- The Biden Administration immediately appealed the decision to the 5th Circuit Court of Appeals.
- April 21: The Biden Administration and Danco Laboratories requested a stay from SCOTUS that would block Kacsmaryk’s decision while litigation played out. SCOTUS granted the stay.
- August 16: The Fifth Circuit rejected and affirmed Kacsmaryk’s decision. The unanimous panel of three judges ruled that the abortion drug mifepristone could remain on the market but would be required to restore the former 2011 safeguards, limiting its use to seven weeks of pregnancy and prohibiting distribution by mail. (See Fifth Circuit 93-page decision here.)
(This ruling did not take effect, however, because of the previously granted stay by SCOTUS.).
- September 8, 2023: The Biden administration and Danco asked SCOTUS to completely overturn the Fifth Circuit decision.
- December 13, 2023: SCOTUS decided to hear both cases. (These are the two cases to be heard by SCOTUS in 2024.) The U.S. Department of Justice is representing the FDA, Hogan Lovells US LLP is representing Danco Laboratories, and the Alliance Defending Freedom is representing the Alliance for Hippocratic Medicine.
Other Cases Involving Mifepristone:
- 18 States Guaranteed Access to Mifepristone
A case filed by 18 state attorneys general was decided the same day as Kacsmaryk’s decision. In this case, State of Washington et al. v. United States Food and Drug Administration et al., Judge Thomas O. Rice issued a preliminary injunction blocking the FDA from limiting access to mifepristone in those 18 states, but he did not address safety precautions related to the drug.
- Three States Filed Lawsuit Against FDA
Missouri Attorney General Andrew Bailey announced on November 6 that another lawsuit had been filed along with a request for it to be merged with the previous lawsuit filed by the Alliance for Hippocratic Medicine. State of Missouri; the State of Kansas; the State of Idaho v. the U.S. Food and Drug Administration; Robert M. Califf M.D., filed in November 6. The lawsuit included a request for an injunction against the 2016 rollback of critical safety standards, the FDA’s 2019 approval of generic mifepristone, and the 2021 and 2023 policy allowing drugs to be sent by mail. The Court was also asked to combine the complaint with the existing lawsuit by AHM to be heard by SCOTUS in 2024.
“Our annual survey findings revealed the consequences of the FDA’s recklessness in lifting nearly every crucial safety standard installed to protect mothers who so greviously seek out abortion through these drugs.” said Operation Rescue President Troy Newman.
“While we continue to fight for the lives of innocent babies, we pray that eyes will be opened, hearts will be softened, souls will be saved, and that our nation’s Highest Court will decide to take vital steps to protect women from an FDA that has made such incredibly reckless, uncaring decisions.”






